GxP Bielefeld 2019
This product is no longer available.
Rapid processing
Secure payment
256 bit encryption
Der Kurs richtet sich an Mitarbeiter/innen, Studenten/innen und Doktoranden/innen aus den Bereichen der Biologie, Biochemie, Biotechnologie, Chemie, Medizin, Pharmazie und Life Science.
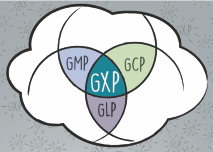
GxP ist ein Sammelbegriff für die Richtlinien der „Guten Arbeitspraxis“. Unsere Weiterbildung lehnt sich an die Anforderungen zur Herstellung von Arzneimitteln. In diesem Kontext soll der Weg von der Entwicklung im Labor, über die Phasen der Zulassung, die Herstellung, bis zur steten Überprüfung auf dem Markt, der Arzneimittelsicherheit, besprochen werden.
Unser GxP-Training umfasst eine Grundlagen-Schulung zu GLP, GCP, GMP und GVP.
• GLP ist ein Qualitätssicherungssystem für die Durchführung von nicht-klinischen Prüfungen von Stoffen zur Überprüfung möglicher Gefahren für Mensch und Umwelt.
• GCP ist die Gute Klinische Praxis. Nach ethischen und wissenschaftlichen Grundsätzen werden die klinischen Studien durchgeführt.
• GMP beschreibt die Gute Herstellungspraxis von Arzneimitteln und ist ein Qualitätssicherungssystem nach vorgeschriebenen Richtlinien.
• GVP ist die Gute Pharmakovigilanz Praxis und steht für die Arzneimittel-Sicherheitsüberwachung nach Marktzulassung gemäß Leitlinien.
Dieser Kurs vermittelt Ihnen Kenntnisse über die Richtlinien und Regelwerke in der pharmazeutischen Industrie. Parallel dazu werden mögliche Berufsfelder vorgestellt, um den Brückenschlag zwischen Hochschule und Jobeinstieg erfolgreich zu meistern.
A cancellation of the course is possible until 29 July 2019. There is a processing fee of 20 €. After the 29 July 2019 is in the individual case only a transfer to a later course possible.
Once the user has seen at least one product this snippet will be visible.